The dynamics of water in proteins is important in determining MRI contrast and the response of protein structure and activity to temperature. We have been studying this problem using a variety of high resolution N-15 filtered MAS methods to monitor cross relaxation between amide H-1 and the H-1 in water contained in nanocrystalline preparations of ubiquitin. The amides do not undergo chemical exchange with the water at any significant rate in these crystals, and the cross relaxation can be modeled assuming only incoherent relaxation proccesses arising from relative motion of the water H-1 relative to the amides. The plot below depicts this fast cross relaxation in an experiment where the initial condition has only the amide H-1s polarized.
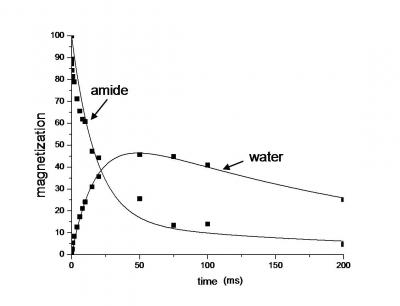
This magnetization decays while that for water grows in due to the efficient cross relaxation. The cross relaxation rate increases with decreasing T, and has an activation energy of ~ 6 kcal/mol, consistent with diffusion in a defect ridden hydrgen bonding network. Current work is aimed at relating the water behavior in crystalline proteins to that in mesoporous solids. Both systems display water freezing point depressions of over 60 K and nanoscopic depression of self diffusion constants. The site specific variability of water dynamics parameters is also being investigated.